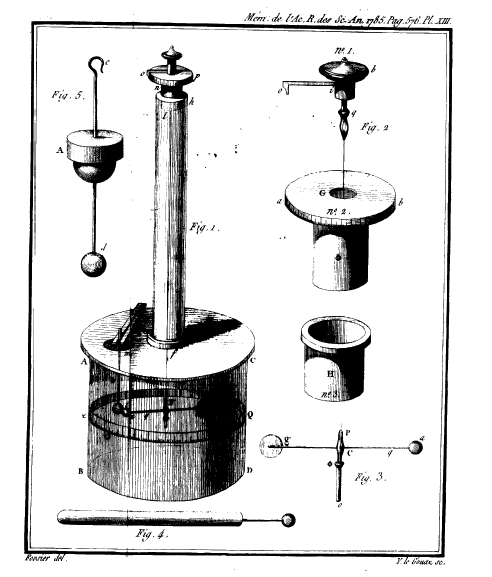
In 1767 Priestley suggested that the strength of electrical attraction varies as the inverse square of the distance. However it was 1785 when the French engineer Charles Augustus Coulomb (1736-1806) proved his assumption.
In 1785 Coulomb presented his three reports on Electricity and Magnetism:
Premier M¨¦moire sur l¡¯Electricit¨¦ et le Magn¨¦tisme. In this publication Coulomb describes "How to construct and use an electric balance (torsion balance) based on the property of the metal wires of having a reaction torsion force proportional to the torsion angle". Coulomb also experimentally determined the law that explains how ¡°two bodies electrified of the same kind of Electricity exert on each other".
S¨¦cond M¨¦moire sur l¡¯Electricit¨¦ et le Magn¨¦tisme. In this publication Coulomb carries out the "determination according to which laws both the Magnetic and the Electric fluids act, either by repulsion or by attraction."
Troisi¨¨me M¨¦moire sur l¡¯Electricit¨¦ et le Magn¨¦tisme. "On the quantity of Electricity that an isolated body loses in a certain time period , either by contact with less humid air, or in the supports more or less idio-electric."
Four subsequent reports were published in the following years:
- Quatri¨¨me M¨¦moire "Where two principal properties of the electric fluid are demonstrated: first, that this fluid does not expand into any object according to a chemical affinity, or by an elective attraction, but that it divides itself between different objects brought into contact; second, that in conducting objects, the fluid, having achieved a state of stability, expands on the surface of the body and does not penetrate into the interior." (1786)
- Cinqui¨¨me M¨¦moire "On the manner in which the electric fluid divides itself between conducting objects brought into contact, and the distribution of this fluid on the different parts of the surface of this object." (1787)
- Sixi¨¨me M¨¦moire "Continuation of research into the distribution of the electric fluid between several conductors. Determination of electric density at different points on the surface of these bodies." (1788)
- Septi¨¨me M¨¦moire. "On magnetism" (1789)"
Here is the date from one of his experiment
| Distant of two ball(Unit) | Torsion's Angle Change (Degree) |
|---|---|
36 |
36 |
18 |
144 |
8.5 |
575.5 |
It shows that: when the ratio of the distance of two charges is 4:2:1, the torsion angle ratio is 1:4:16, which is satisfied with the inverse square rule. The last pair of data does not fit well, which Coulomb believed it was caused by electric leakage.
Quiz
At that time, people had no way to measure charge. How did Coulomb know the charge of a ball?
Answer
The answer is Coulomb didn't know the charge of the ball. He just knew the ratio. For example, at first he put some charge on the metal ball, after measuring the distance and torsion angle. He contacted the ball with another same size metal ball, so that he got the half charge of the original one. Coulomb didn't explain the reason why it works. Now we can say since two metal balls connect together, they have the same potential. And potential is just the function of size and charge. Since they have the same size and the total charge will never change, each ball has half of the original charge.

Coulomb's torsion balance
This picture comes from: http://en.wikipedia.org/wiki/Image:Bcoulomb.png
Reference
1.http://en.wikipedia.org/wiki/Charles-Augustin_de_Coulomb